Is decrease in enthalpy a criterion for spontaneity?
`=>` If we examine the phenomenon like flow of water down hill or fall of a stone on to the ground, we find that there is a net decrease in potential energy in the direction of change.
`=>` By analogy, we may be tempted to state that a chemical reaction is spontaneous in a given direction, because decrease in energy has taken place, as in the case of exothermic reactions.
● For example :
`color{purple}(1/2 N_2 (g) +3/2 H_2 (g) = NH_3 (g) ; Delta_r H^(⊖) = - 46.1 kJ mol^(-1))`
`color{purple}(1/2 H_2 (g) +1/2 Cl_2 (g) = HCl (g) ; Delta_r H^(⊖) = -92.32 kJ mol^(-1))`
`color{purple}(H_2 (g) +1/2 O_2 (g) → H_2O (l) ; Delta_r H^(⊖) = -285.8 kJ mol^(-1))`
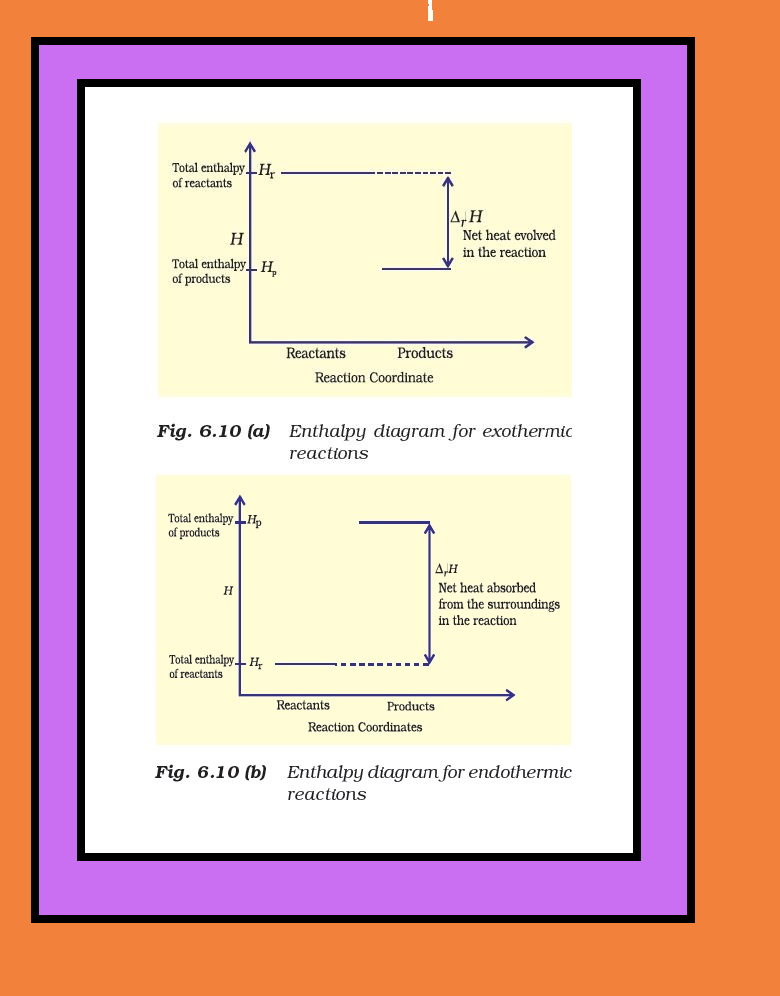
`=>` The decrease in enthalpy in passing from reactants to products may be shown for any exothermic reaction on an enthalpy diagram as shown in Fig. 6.10(a).
●Thus, the postulate that driving force for a chemical reaction may be due to decrease in energy sounds ‘reasonable’ as the basis of evidence so far.
`=>` Now let us examine the following reactions :
`color{purple}(1/2 N_2 (g) +O_2 (g) → NO_2 (g) ; Delta_r H^(⊖) = +32.2 kJ mol^(-1))`
`color{purple}(C_text{(graphite, s)} + 2 S(l) → CS_2(l); Delta_r H^(⊖) = +128.5 kJ mol^(-1))`
● These reactions though endothermic, are spontaneous.
● The increase in enthalpy may be represented on an enthalpy diagram as shown in Fig. 6.10(b).
`=>` Therefore, it becomes obvious that while decrease in enthalpy may be a contributory factor for spontaneity, but it is not true for all cases.
`=>` By analogy, we may be tempted to state that a chemical reaction is spontaneous in a given direction, because decrease in energy has taken place, as in the case of exothermic reactions.
● For example :
`color{purple}(1/2 N_2 (g) +3/2 H_2 (g) = NH_3 (g) ; Delta_r H^(⊖) = - 46.1 kJ mol^(-1))`
`color{purple}(1/2 H_2 (g) +1/2 Cl_2 (g) = HCl (g) ; Delta_r H^(⊖) = -92.32 kJ mol^(-1))`
`color{purple}(H_2 (g) +1/2 O_2 (g) → H_2O (l) ; Delta_r H^(⊖) = -285.8 kJ mol^(-1))`
`=>` The decrease in enthalpy in passing from reactants to products may be shown for any exothermic reaction on an enthalpy diagram as shown in Fig. 6.10(a).
●Thus, the postulate that driving force for a chemical reaction may be due to decrease in energy sounds ‘reasonable’ as the basis of evidence so far.
`=>` Now let us examine the following reactions :
`color{purple}(1/2 N_2 (g) +O_2 (g) → NO_2 (g) ; Delta_r H^(⊖) = +32.2 kJ mol^(-1))`
`color{purple}(C_text{(graphite, s)} + 2 S(l) → CS_2(l); Delta_r H^(⊖) = +128.5 kJ mol^(-1))`
● These reactions though endothermic, are spontaneous.
● The increase in enthalpy may be represented on an enthalpy diagram as shown in Fig. 6.10(b).
`=>` Therefore, it becomes obvious that while decrease in enthalpy may be a contributory factor for spontaneity, but it is not true for all cases.